Para proteger os consumidores de medicamentos falsificados, contaminados ou adulterados, impróprios para distribuição nos EUA, o Congresso dos EUA promulgou a Lei de Segurança da Cadeia de Suprimentos de Medicamentos (DSCSA) em 2013. A conformidade com a DSCSA garante que todos os participantes da cadeia de suprimentos farmacêuticos atendam aos rigorosos requisitos da DSCSA para farmácias e outras entidades, melhorando a segurança geral.
A DSCSA exige que cada embalagem de medicamento de prescrição e caixa homogênea tenha seu próprio identificador exclusivo em formas legíveis por máquina e por humanos. Esses requisitos farmacêuticos de conformidade com a DSCSA incluem:
Além disso, cada identificador deve ser armazenado em um código de barras 2D Datamatrix, dando suporte à interoperabilidade eletrônica para rastrear produtos de medicamentos prescritos em toda a cadeia de suprimentos.
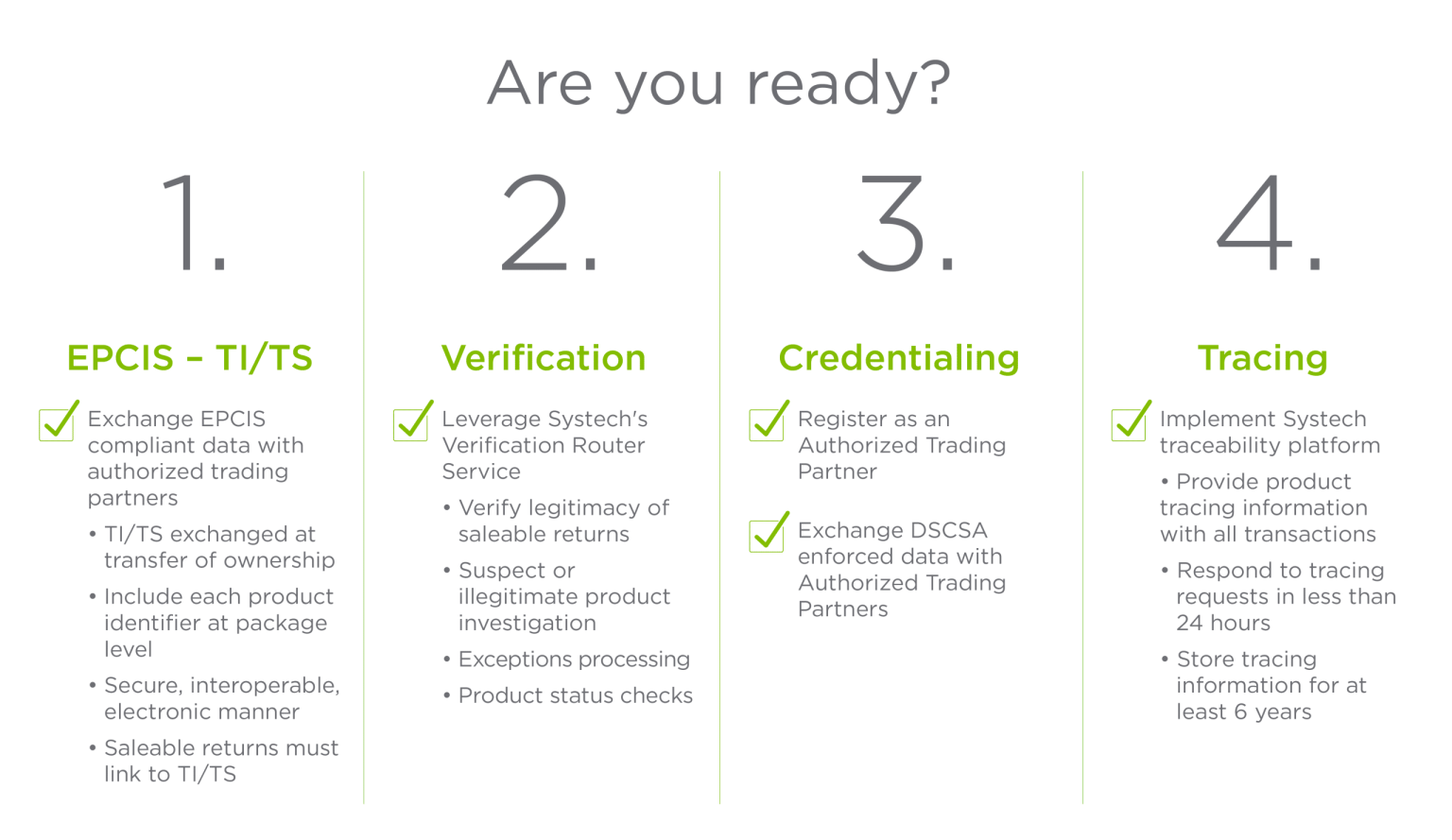
Embora o prazo oficial da DSCSA para conformidade com a interoperabilidade - 27 de novembro de 2023 - já tenha passado, a FDA recentemente introduziu isenções recentes na forma de um plano de implementação em fases para a DSCSA. Durante esse período, é fundamental que as farmácias e outras entidades da cadeia de suprimentos atendam aos requisitos da DSCSA para produtos farmacêuticos e garantam a conformidade antes do prazo estendido. Como líder global em serialização, a Systech oferece a velocidade, a flexibilidade e a experiência comprovada necessárias para que você esteja em conformidade. Juntos, implementaremos soluções.
Implementar gerenciamento de exceções para garantir que o físico corresponda ao digital.
Como líder em serialização, a Systech oferece um serviço completo de rastreabilidade solução para todos no ecossistema da cadeia de suprimentos - fabricantes, empacotadores contratados, distribuidores, 3PLsdispensários e órgãos reguladores. Nosso processo de implementação comprovado é configurável, dimensionável e desenvolvido para atender aos requisitos de integração e conformidade de hoje e de amanhã.

A Systech tem orgulho de ser reconhecida como um provedor de VRS comprometido com a Iniciativa de credenciamento aberto
"A OCI é uma iniciativa aberta que permite interações digitais confiáveis entre Parceiros Comerciais Autorizados (ATPs) na cadeia de suprimentos farmacêutica dos EUA para atender aos requisitos da Lei de Segurança da Cadeia de Suprimentos de Medicamentos dos EUA (DSCSA)."
Conclusões da recente Conferência Anual da FDLI de 2024 com foco na preparação para a DSCSA...
Insights sobre a situação atual e as oportunidades oferecidas pelo período de estabilização do DSCSA....
Como parte da Lei de Qualidade e Segurança de Medicamentos da FDA, uma das principais justificativas para a Lei de Segurança de Fornecimento de Medicamentos (DSCSA)...