The fastest way to secure compliance and visibility in the pharmaceutical supply chain starts with UniTrace.
Systech’s cloud-based platform simplifies pharmaceutical track and trace with full serialization and end-to-end traceability from manufacturing to dispensing.
Trusted by leading companies like Nutra-Med Packaging, UniTrace helps you move faster, connect easier and ensure compliance at every step.
“UniTrace makes it faster, easier and more reliable to connect with our customers.”
— Kunal Gupta, CEO, Nutra–Med Packaging
A complete, single-vendor pharmaceutical track and trace solution for trade partner data exchange and seamless exception management.
Focused on standards-based implementation and industry-leading service, speeding up your deployment process.
Built on EPCIS standards to support global regulatory requirements with seamless and accurate data exchange.
Upfront scope and transparent pricing with no hidden costs for enterprise-grade performance.
UniTrace brings the entire supply chain together—manufacturers, contract packagers, distributors, logistics providers and pharmacies. The first fully integrated pharmaceutical track and trace solution of its kind, UniTrace delivers fast implementation and streamlined compliance.
Built on EPCIS standards, UniTrace supports all major global regulations—including DSCSA and EU FMD—and includes a built-in data checker module for accurate data exchange across your network.
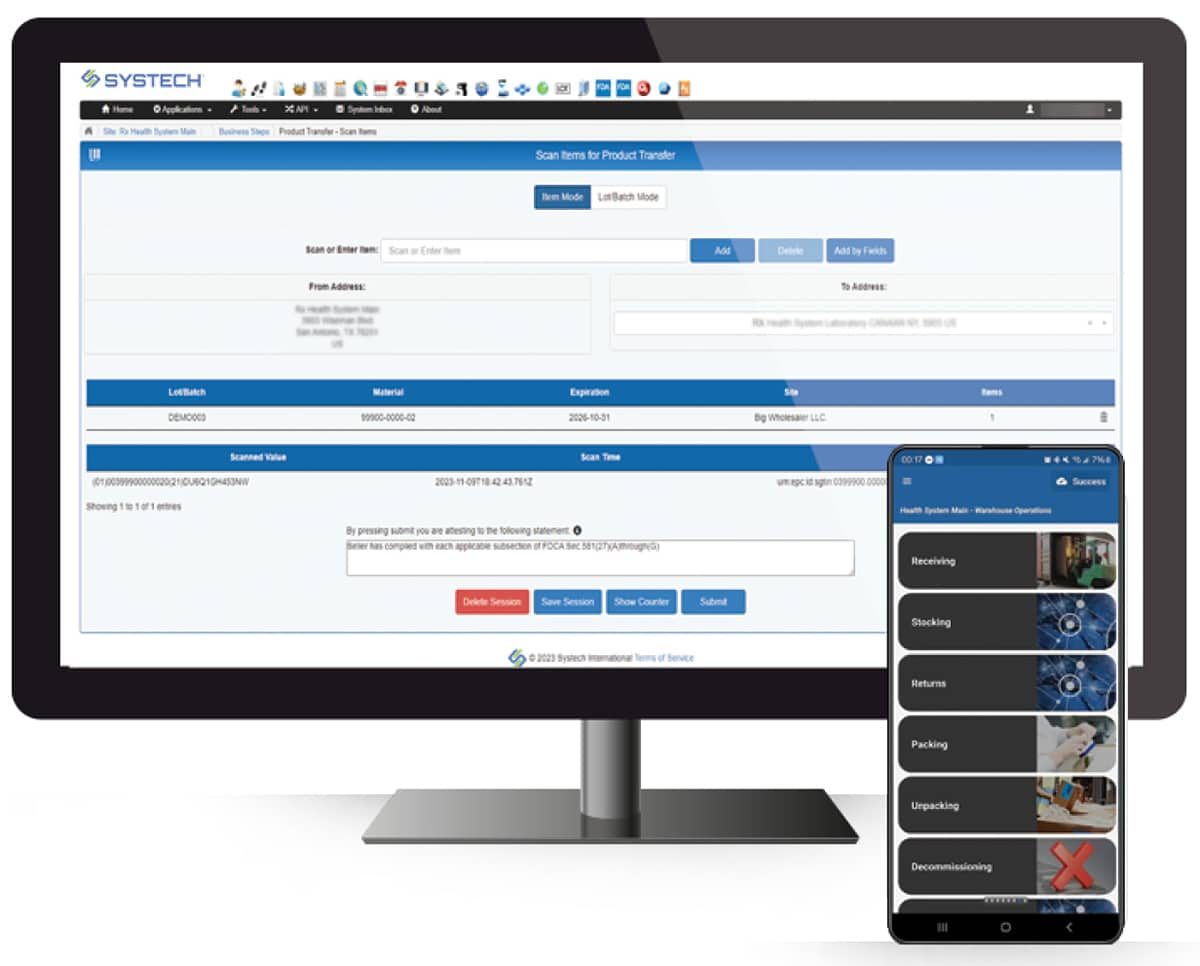
Go beyond basic pharmaceutical track and trace with Systech Exception Manager, resolving issues before they slow you down.
UniTrace Mobile puts full pharmaceutical track and trace capabilities in the palm of your hand. From creating shipping, receiving and return events to managing recalls, expirations, QA release and internal transfers—you can do it all, anywhere.
Our multi-scan technology is built for efficiency in high-throughput environments and can be easily integrated on a mobile or industrial device.
Learn how Systech helped mitigate EPCIS delays and compliance risk…
Learn how using open global and proven standards can improve pharma supply chain…
Discover why Nutra-Med made the strategic decision to partner with Systech…