Pour protéger les consommateurs contre les médicaments de prescription contrefaits, contaminés ou falsifiés, impropres à la distribution aux États-Unis, le Congrès américain a promulgué la loi sur les médicaments de prescription. Loi sur la sécurité de la chaîne d'approvisionnement en médicaments (DSCSA) en 2013. La conformité à la DSCSA garantit que tous les acteurs de la chaîne d'approvisionnement pharmaceutique respectent les exigences strictes de la DSCSA pour les pharmacies et les autres entités, ce qui renforce la sûreté et la sécurité globales.
La DSCSA exige que chaque emballage de médicament sur ordonnance et chaque étui homogène ait son propre identifiant unique sous des formes lisibles par la machine et par l'homme. Ces exigences de conformité DSCSA pour les produits pharmaceutiques sont les suivantes :
En outre, chaque identifiant doit être stocké dans un code-barres Datamatrix 2D, ce qui favorise l'interopérabilité électronique pour tracer les médicaments sur ordonnance tout au long de la chaîne d'approvisionnement.
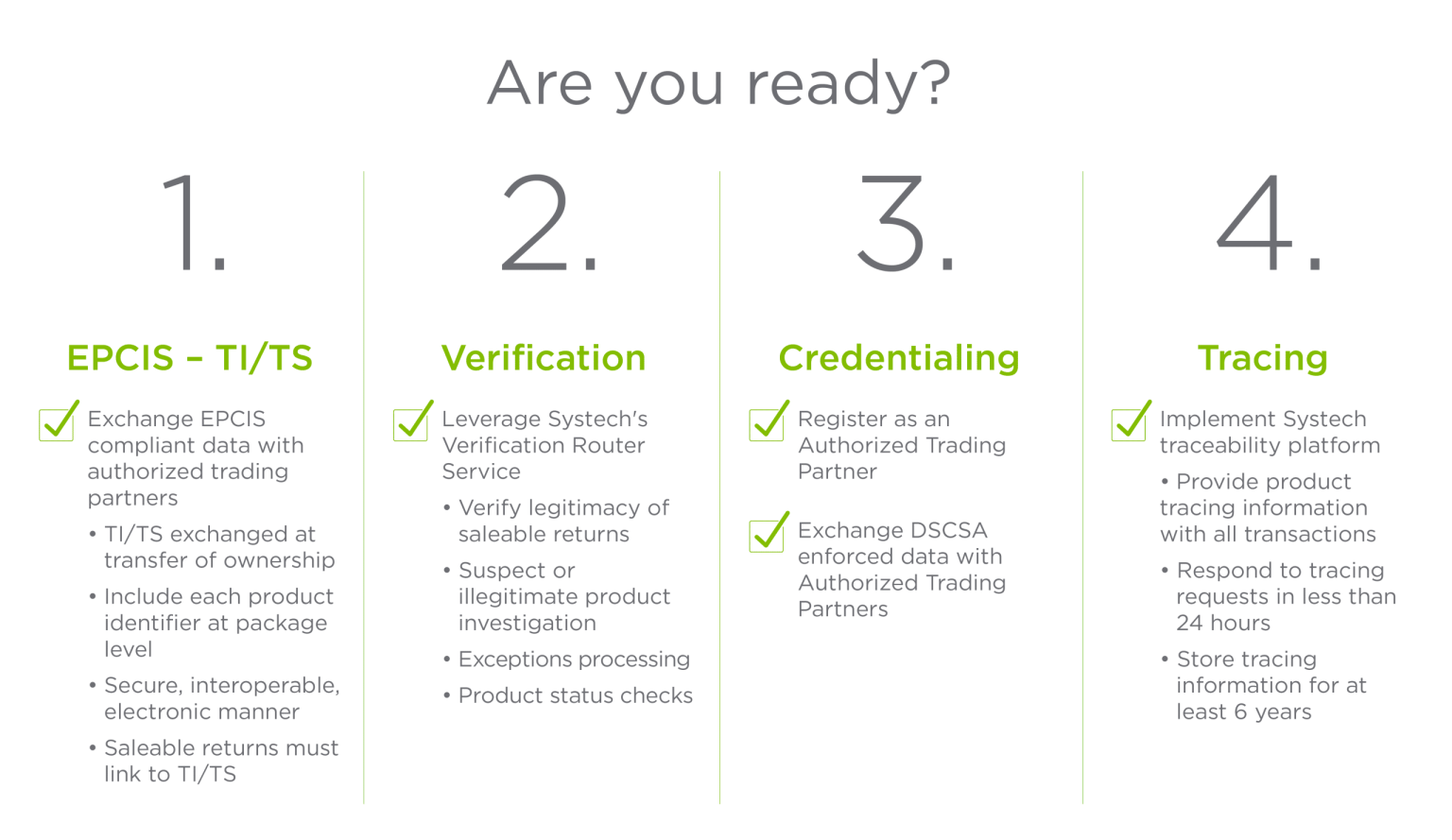
Bien que l'échéance officielle de la DSCSA pour la conformité à l'interopérabilité - le 27 novembre 2023 - soit dépassée, la DSCSA n'est pas encore entrée en vigueur. FDA a récemment introduit des exemptions sous la forme de un plan de mise en œuvre progressive de la DSCSA. Pendant cette période, il est crucial que les pharmacies et autres entités de la chaîne d'approvisionnement répondent aux exigences de la DSCSA pour les produits pharmaceutiques et assurent la conformité avant la date limite prolongée. En tant que leader mondial de la sérialisation, Systech offre la rapidité, la flexibilité et l'expertise éprouvée nécessaires pour vous mettre en conformité. Ensemble, nous mettrons en œuvre des solutions.
Mettre en œuvre gestion des exceptions pour que le physique corresponde au numérique.
En tant que leader dans le domaine de la sérialisation, Systech offre une gamme complète de services de sérialisation. traçabilité pour tous les acteurs de l'écosystème de la chaîne d'approvisionnement - fabricants, conditionneurs à façon, distributeurs, 3PLLes services de santé, les dispensaires et les organismes de réglementation. Notre processus de mise en œuvre éprouvé est configurable, évolutif et conçu pour répondre aux exigences d'intégration et de conformité d'aujourd'hui et de demain.

Systech est fier d'être reconnu comme un fournisseur de VRS qui s'engage à respecter les droits de l'homme. L'initiative pour la reconnaissance des titres de compétences en libre accès
"L'OCI est une initiative ouverte permettant des interactions numériques de confiance entre les partenaires commerciaux autorisés (ATP) dans la chaîne d'approvisionnement pharmaceutique américaine afin de répondre aux exigences de la loi américaine sur la sécurité de la chaîne d'approvisionnement pharmaceutique (DSCSA)".
Les enseignements de la récente conférence annuelle 2024 de la FDLI, avec un accent particulier sur la préparation à la DSCSA...
Aperçu de la situation actuelle et des possibilités offertes par la période de stabilisation de l'ASDC....
Dans le cadre de la loi sur la qualité et la sécurité des médicaments (Drug Quality and Security Act) de la FDA, l'une des principales raisons d'être de la loi sur la sécurité de l'approvisionnement en médicaments (DSCSA)...