Um die Verbraucher vor gefälschten, verunreinigten oder verfälschten verschreibungspflichtigen Medikamenten zu schützen, die nicht für den Vertrieb in den USA geeignet sind, hat der US-Kongress das des Gesetzes zur Sicherheit der Arzneimittelversorgungskette (DSCSA) an die erweiterte Sicherheit des Arzneimittelvertriebs im Jahr 2013. Die Einhaltung des DSCSA gewährleistet, dass alle Akteure in der pharmazeutischen Lieferkette die strengen DSCSA-Anforderungen für Apotheken und andere Einrichtungen erfüllen, was die Sicherheit insgesamt erhöht.
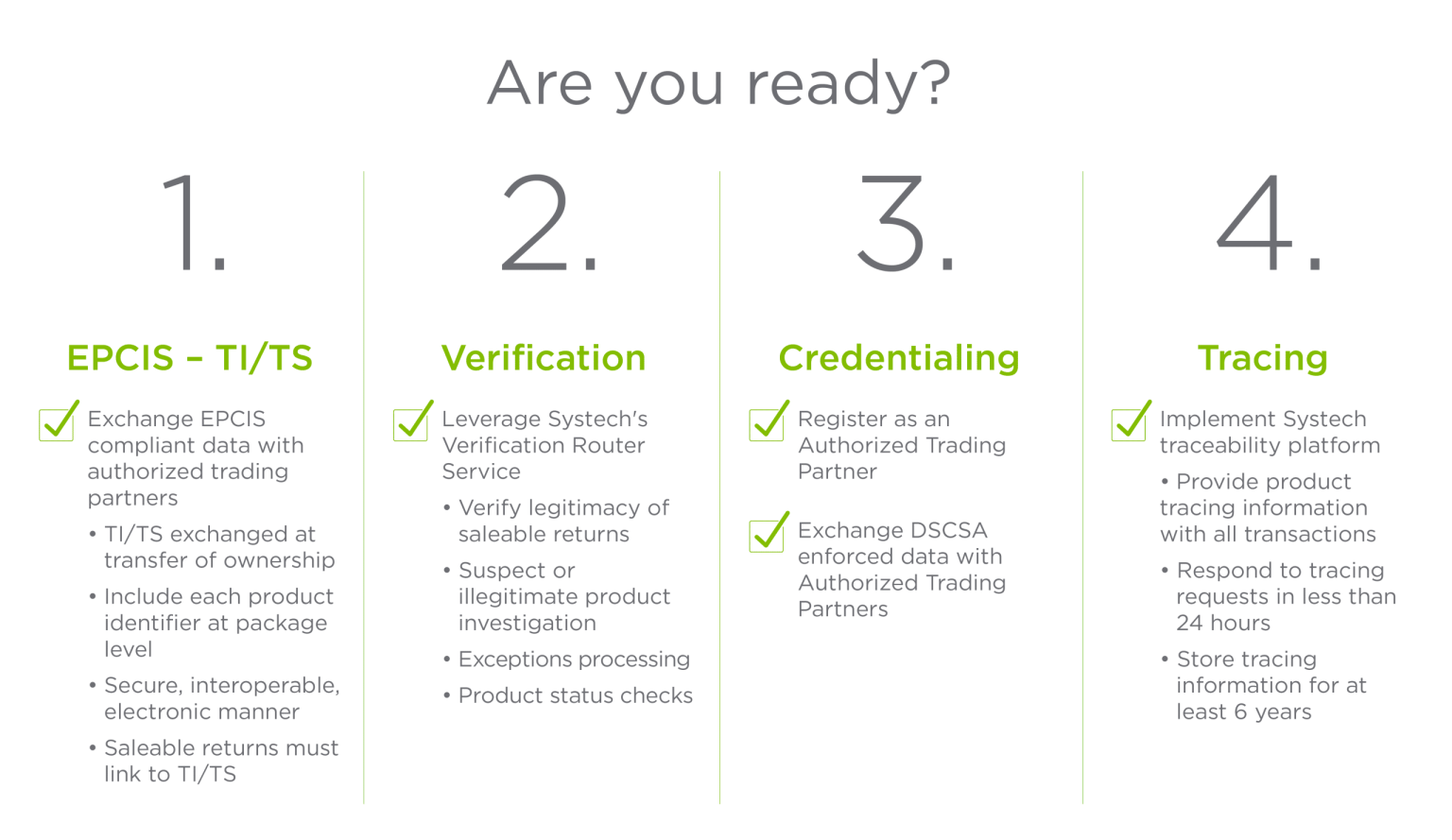
Der DSCSA schreibt vor, dass jede verschreibungspflichtige Arzneimittelpackung und jede homogene Packung eine eigene eindeutige Kennung in maschinen- und menschenlesbarer Form haben muss. Diese DSCSA-Compliance-Anforderungen für die Pharmaindustrie umfassen:
Darüber hinaus muss jeder Identifikator in einem 2D-Datamatrix-Barcode gespeichert werden, der die elektronische Interoperabilität zur Rückverfolgung von verschreibungspflichtigen Arzneimitteln in der gesamten Lieferkette unterstützt.
Obwohl die offizielle DSCSA-Frist für die Einhaltung der Interoperabilität - der 27. November 2023 - bereits verstrichen ist, ist die FDA hat in jüngster Zeit Ausnahmeregelungen eingeführt in Form von einen stufenweisen Umsetzungsplan für DSCSA. In diesem Zeitraum ist es von entscheidender Bedeutung, dass Apotheken und andere Unternehmen der Lieferkette die DSCSA-Anforderungen für Arzneimittel erfüllen und die Einhaltung der Vorschriften vor Ablauf der verlängerten Frist sicherstellen. Als weltweit führender Anbieter von Serialisierungslösungen bietet Systech die Schnelligkeit, Flexibilität und bewährte Fachkompetenz, die Sie benötigen, um die Anforderungen zu erfüllen. Gemeinsam werden wir Lösungen implementieren.
Umsetzung Ausnahmemanagement Lösung, um sicherzustellen, dass das Physische mit dem Digitalen übereinstimmt.
Als führendes Unternehmen im Bereich der Serialisierung bietet Systech ein komplettes Rückverfolgbarkeit Lösung für alle Beteiligten der Lieferkette - Hersteller, Lohnverpacker, Händler, 3PLs, Apotheken und Aufsichtsbehörden. Unser bewährter Implementierungsprozess ist konfigurierbar, skalierbar und so aufgebaut, dass er die Integrations- und Compliance-Anforderungen von heute und morgen erfüllt.

Systech ist stolz darauf, als VRS-Anbieter anerkannt zu sein, der sich für die Initiative zur offenen Akkreditierung
"OCI ist eine offene Initiative, die vertrauenswürdige digitale Interaktionen zwischen autorisierten Handelspartnern (ATPs) in der pharmazeutischen Lieferkette in den USA ermöglicht, um die Anforderungen des U.S. Drug Supply Chain Security Act (DSCSA) zu erfüllen."
Eindrücke von der jüngsten FDLI-Jahreskonferenz 2024 mit Schwerpunkt auf der Vorbereitung auf DSCSA...
Einblicke in den aktuellen Stand und die Möglichkeiten der DSCSA-Stabilisierungsphase....
Als Teil des Drug Quality and Security Act der FDA ist eines der Hauptargumente für den Drug Supply Security Act (DSCSA)...